Click here to see all images
June, 2024
Case of the Month
Clinical History:
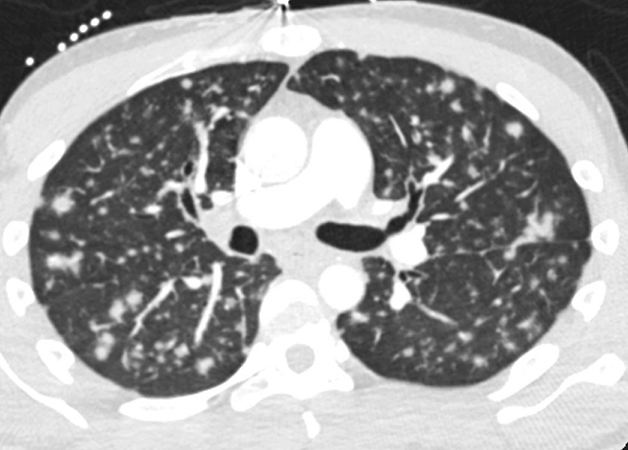
A 30-year-old man with a remote history of HIV/AIDS, subsequently lost to follow up presented with a 3-week history of sore throat and coughing with occasional hemoptysis. Physical exam was normal. Chest CT scan showed innumerable bilateral pulmonary nodules 6-9 mm in size (Figure 1). A transbronchial biopsy of the right lower lobe was performed for further evaluation. Microscopically, there were multiple pieces of unremarkable lung parenchyma with only two pieces involved by a monotonous, low-grade spindle cell proliferation with focal fascicular growth pattern (Figure 2). The lesional process also formed vascular spaces, with some having a “slit-like” appearance containing extravasated red blood cells (Figure 3). Rare cytoplasmic hyaline globules were seen. No organizing pneumonia or granulomas were identified. GMS and AFB special stains were negative for fungi and acid-fast organisms, respectively. Immunohistochemical stains showed that the spindle cell proliferation was positive for D2-40 (Figure 4), ERG (Figure 5), and CD34 (focal and weak), while negative for pan-CK and CD68. The spindle cells were positive for HHV-8 in a nuclear speckled pattern (Figure 6). CMV immunostaining was negative.
Q1. What is the diagnosis?
- Angiosarcoma
- Epithelioid hemangioendothelioma
- Kaposi sarcoma
- Mycobacterial spindle cell tumor
Q2. What is the most essential and helpful ancillary test to confirm this diagnosis?
- HHV-8
- ERG
- D2-40
- GLUT1
Q3. Which of the following is true about this entity?
- This condition may be cured by excision and neoadjuvant therapy
- It can present in either the patch stage, plaque stage, or tumor stage
- Multiple lesions may arise in different sites and indicate metastatic spread
- It is not classically associated with immunosuppression
Answers to Quiz
Q2. A
Q3. B
Diagnosis
Discussion
Pulmonary involvement by KS can be seen in as many as 6-30% of AIDS patients with cutaneous lesions and in 40-75% of these patients at autopsy. However, pulmonary involvement without cutaneous lesions is quite rare. In this setting, patients present with non-specific respiratory symptoms as the ones described in the case (cough, shortness of breath, hemoptysis). The lungs may be involved at any level with often endobronchial lesions seen as red-purple macules or papules on bronchoscopy. Chest HRCT scan demonstrates peribronchovascular and interlobular septal thickening; bilateral and symmetric ill-defined nodules in a peribronchovascular distribution; and fissural nodularity. The diagnosis of pulmonary KS may be suspected based on the integration of the clinical context and imaging findings, however, histopathologic confirmation is required for diagnosis in an endobronchial or transbronchial biopsy.
Histologically, pulmonary KS is similar to its counterpart in the skin, but involvement of the lung can be subtle and easily overlooked since the presence of interconnected vascular channels may be difficult to distinguish from artefactual collapse of normal or reactive lung parenchyma. Lesions with extensive vascular proliferation may be more obvious. KS is composed of a spindle cell proliferation with mild to moderate nuclear atypia, forming “slit-like” vascular spaces variably filled with red blood cells. Extravasated red cells and hyaline globules (degenerated red cells) are also a common feature, but they may not be obvious in a small biopsy.
The differential diagnosis of pulmonary KS includes other vascular entities such as low-grade angiosarcoma, epithelioid hemangioendothelioma, and mycobacterial spindle cell tumor. Morphologically, low-grade angiosarcoma shows anastomosing vascular channels, occasional nuclear hobnailing, but does not contain spindle cells or hyaline globules. Epithelioid hemangioendothelioma shows intra-alveolar growth, epithelioid -not spindle- morphology and the formation of a chondromyxoid matrix. Mycobacterial spindle cell tumor is composed of sheets of bland spindle cells but it does not feature vascular spaces and it contains cells with foamy cytoplasm. By immunohistochemistry, low-grade angiosarcoma and epithelioid hemangioendothelioma share the expression of vascular markers with KS (CD31, CD34, ERG), but by definition they are negative for HHV-8. Epithelioid hemangioendothelioma is also positive for cytokeratin and either nuclear CAMTA in cases with the WWTR1-CAMTA1 fusion, or TFE3 in cases with the YAP1-TFE3 fusion. In mycobacterial spindle cell tumor, acid-fast stains highlight the presence of numerous bacilli and by immunohistochemistry the spindle cells are positive for CD68 and CD163 and negative for vascular markers and HHV-8. Therefore, HHV-8 immunohistochemistry is a crucial ancillary test to confirm the diagnosis of KS.
Treatment of KS may include chemotherapy, radiation, and antiviral medication. Overall, prognosis is poor for patients with widespread disease. However, in patients with pulmonary involvement, studies have shown marked improvement in clinical symptoms and pulmonary function when combination chemotherapy is utilized.
Take-home message for trainees: Kaposi sarcoma is a low-grade vascular neoplasm that is associated with HHV-8 infection and an immunocompromised status, usually HIV/AIDS. Diagnostic features in the lung may be subtle by morphology and a confirmatory diagnosis requires a high index of suspicion and positivity for HHV-8 by immunohistochemistry.
References
Gasparetto TD, Marchiori E, Lourenço S, et al. Pulmonary involvement in Kaposi sarcoma: correlation between imaging and pathology. Orphanet J Rare Dis. 2009; Jul 14;4:18. doi: 10.1186/1750-1172-4-18. PMID: 19602252; PMCID: PMC2720383.
Meduri GU, Stover DE, Lee M, et al. Pulmonary Kaposi's sarcoma in the acquired immune deficiency syndrome. Clinical, radiographic, and pathologic manifestations. Am J Med. 1986; Jul;81(1):11-8. doi: 10.1016/0002-9343(86)90175-0. PMID: 3728535.
Ramos AL, Granado J, Calderón AI, et al. Pulmonary Kaposi's sarcoma-an atypical clinical presentation. Int J Infect Dis. 2022 Feb;115:185-188. doi: 10.1016/j.ijid.2021.11.031. Epub 2021; Nov 26. PMID: 34843958.Contributors
AP/CP Pathology Resident, PGY-3
Department of Pathology
Duke University Hospital
Durham, NC
Sergio Pina-Oviedo, MD
Associate Professor
Department of Pathology
Duke University Hospital
Durham, NC
Elizabeth Pavlisko, MD
Associate Professor
Department of Pathology
Duke University Medical Center
Durham, NC
John Carney, MD
Assistant Professor
Department of Pathology
Duke University Medical Center
Durham, NC
Huihua Li, MD
Assistant Professor
Department of Pathology
Duke University Medical Center
Durham, NC
Victor Roggli, MD
Professor
Department of Pathology
Duke University Medical Center
Durham, NC
Carolyn Glass, MD, PhD
Associate Professor
Department of Pathology
Duke University Medical Center
Durham, NC
Lou DiBernardo, MD
Assistant Professor
Department of Pathology
Duke University Medical Center
Durham, NC